Chemistry, 24.09.2021 04:30 KArrington815
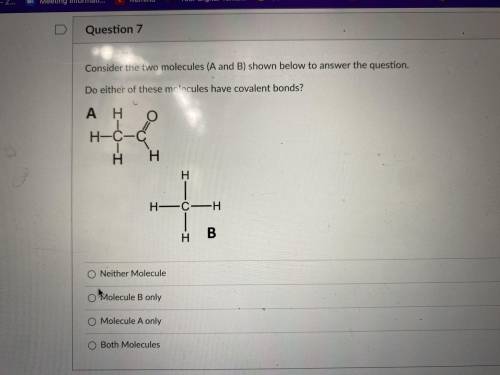
Consider the two molecules (A and B) shown Below to answer the question. Do either of these molecules have covalent bonds ?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:00, kcarstensen59070
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1

Chemistry, 23.06.2019 03:00, rayne40
The size (radius) of an oxygen molecule is about 2.0 ×10−10m. make a rough estimate of the pressure at which the finite volume of the molecules should cause noticeable deviations from ideal-gas behavior at ordinary temperatures (t= 300k ). assume that deviatons would be noticeable when volume of the gas per molecule equals the volume of the molecule itself.
Answers: 3


Chemistry, 23.06.2019 11:00, jdisalle2808
Achemist weighed out 101.g of silver. calculate the number of moles of silver she weighed out.
Answers: 2
You know the right answer?
Consider the two molecules (A and B) shown Below to answer the question. Do either of these molecule...
Questions in other subjects:

Social Studies, 25.07.2019 16:30


History, 25.07.2019 16:30

Chemistry, 25.07.2019 16:30

Mathematics, 25.07.2019 16:30



Social Studies, 25.07.2019 16:30

Biology, 25.07.2019 16:30