Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 17:10, glitterpanda2468
Calculate the estimated density of each ball. use the formula d = m/v where d is the density, m is the mass, and v is the volume. record your calculations in table a of your student guide. given that the density of water is 1.0 g/cm3, make a prediction about whether each ball will float in water. record your prediction in table a. what is the estimated density of the table tennis ball? record your answer to the nearest hundredth
Answers: 2

Chemistry, 22.06.2019 17:30, Naysa150724
Oil rich countries in the middle east cover about 4% of earths total land area but prossess about 48% of the worlds known oil reserves what is the main reason for high concentration of reserves in this part of the world
Answers: 3

Chemistry, 22.06.2019 17:40, aguilarjose
If 3 moles of a compound use 24 j of energy in a reaction, what is the a hreaction in j/mol?
Answers: 1
You know the right answer?
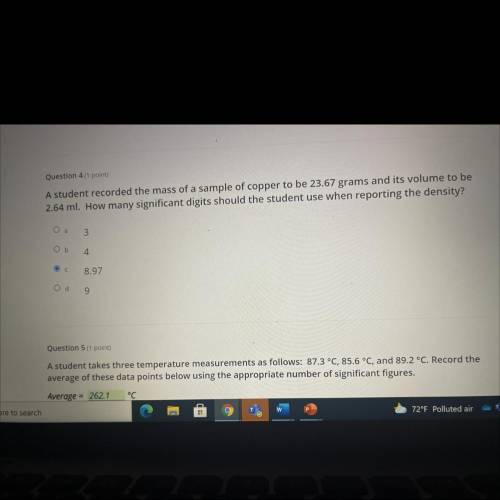
A student recorded the mass of a sample of copper to be 23.67 grams and its volume to be
2.64 ml....
Questions in other subjects:

Mathematics, 24.03.2021 03:50


History, 24.03.2021 03:50

Mathematics, 24.03.2021 03:50




Social Studies, 24.03.2021 03:50


Mathematics, 24.03.2021 03:50