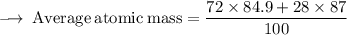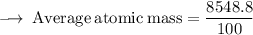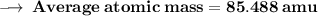Chemistry, 22.09.2021 14:00 demetricejames
Element X has two isotopes. If 72.0% of the element has an isotope mass of 84.9 atomic mass units, and 28.0% of the element has an isotopic mass of 87.0 atomic mass units, the average atomic mass of element X is numerically equal to

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 12:40, vanessa23272
How can you increase the ability of a gas to dissolve in a liquids?
Answers: 1

Chemistry, 22.06.2019 19:00, miguel454545
Avolleyball player hit a ball with a mass of 0.25 kg. the average acceleration of the ball is 15.5 m/s². how much force did the volleyball player apply to the ball? 62.0 n 3.87 n 62.0 m/s² 3.87 m/s²
Answers: 2


Chemistry, 23.06.2019 01:30, oliviacolaizzi
What happens to the concentration of hydronium ions as the ph of a solution increases? a. hydronium ion concentration stays the same b. hydronium ion concentration decreases c. hydronium ion concentration increases
Answers: 1
You know the right answer?
Element X has two isotopes. If 72.0% of the element has an isotope mass of 84.9 atomic mass units, a...
Questions in other subjects: