
Chemistry, 22.09.2021 02:30 gwendallinesikes
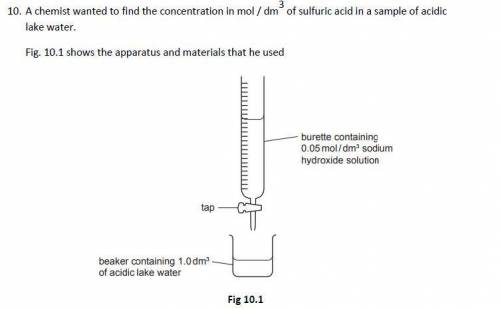
The chemist slowly added 0.05 mol / dm3 sodium hydroxide solution to 1.0 dm3 of acidic lake water contained in a beaker until the acid had just been neutralised
The chemist found that it required 12.5 cm3 of 0.05 mol / dm3 sodium hydroxide solution to neutralise the acid
a. State the number of moles of sodium hydroxide which are dissolved in 1.0 dm3 of the sodium hydroxide solution
b. Calculate the number of moles of sodium hydroxide which are dissolved in 12.5 cm3 of the sodium hydroxide solution. Show your workings.
Show your working.
c. The balanced equation for the neutralisation reaction is
2NaOH + H2SO4 → Na2SO4 + 2H2O
Calculate the number of moles of sulfuric acid which were contained in 1.0 dm3 of acidic lake water.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:30, maddietomlinson113
If the element whose electric configuration ends in the d sublevel, the element is calssified as? a. inner transition b. noble gases c. representative d. transition
Answers: 2

Chemistry, 22.06.2019 09:30, raizagisselle1694
Mike and mitchell decide to have a foot race. they mark off a stretch of 100 yards, and recruit cindy to work the stopwatch. after running the race and looking at the results, cindy declared that mitchell was the fastest. so how did the boys times compare?
Answers: 3


Chemistry, 22.06.2019 20:30, Schoolworkspace453
Consider the following unbalanced equation for the combustion of hexane: αc6h14(g)+βo2(g)→γco2(g)+δh2o(g) part a balance the equation. give your answer as an ordered set of numbers α, β, γ, use the least possible integers for the coefficients. α α , β, γ, δ = nothing request answer part b determine how many moles of o2 are required to react completely with 5.6 moles c6h14. express your answer using two significant figures. n n = nothing mol request answer provide feedback
Answers: 2
You know the right answer?
The chemist slowly added 0.05 mol / dm3 sodium hydroxide solution to 1.0 dm3 of acidic lake water co...
Questions in other subjects:

Mathematics, 24.06.2019 10:00








Social Studies, 24.06.2019 10:00



