Questions
Q10 Changes in attractive forces when water vaporizes
Which of the following descr...

Chemistry, 21.09.2021 19:40 pulverjonathan1
Questions
Q10 Changes in attractive forces when water vaporizes
Which of the following describes the changes in forces of attraction that occur as H20 changes phase from a liquid to a vapor?
(A
H-O bonds break as H-Hand O- bonds form.
B
Hydrogen bonds between H20 molecules are broken.
C
Covalent bonds between H20 molecules are broken.
D
Ionic bonds between Htions and OH" ions are broken.
(E
Covalent bonds between H ions and H20 molecules become more effective.
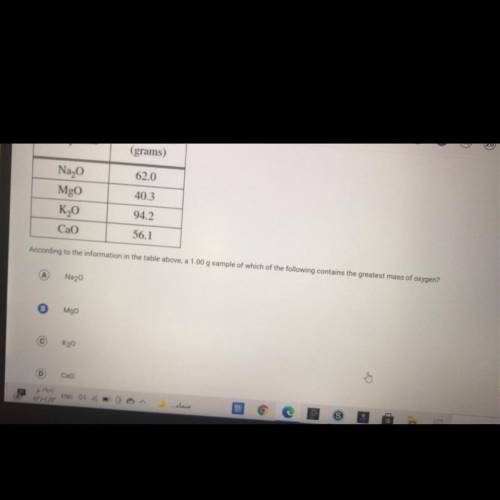

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:00, dayjionneam
In the analysis of hair and fiber samples, which does a compound comparison microscope allow for that a conventional compound microscope does not? a. simultaneous observation b. polarization c. fluorescence d. higher magnification
Answers: 2

Chemistry, 22.06.2019 16:30, ddmoorehouseov75lc
Correct relationship between molecular formula and empirical formula
Answers: 1

Chemistry, 22.06.2019 18:00, jeepjose58
What amount of heat is exchanged when 106.2 grams of substance y goes from a liquid at 35 degrees celsius to a solid at the same temperature? melting point of substance y = 35 degrees c; δhvaporization = 3.67 j/mol; δhfusion = 3.30 j/mol. mwsubstance y = 28.22 g/mol. −12.4 j −3.51 x 102 j 1.24 x 101 j 351 j
Answers: 1
You know the right answer?
Questions in other subjects:

Social Studies, 29.09.2019 20:30


Mathematics, 29.09.2019 20:30






Mathematics, 29.09.2019 20:30



