
Chemistry, 18.09.2021 01:00 annemcnair217
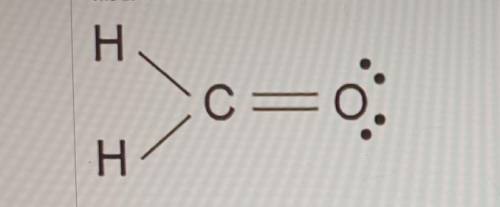
The Lewis dot model of a molecule is shown. Based on the model, which of the following is true? (5 points)
O Oxygen is the least electronegative of the three atoms.
O Carbon has a total of four bonded pairs of electrons around it.
O Oxygen has four pairs of non-bonding innermost shell electrons.
O Carbon has an incomplete octet as it transfers an electron to each hydrogen.

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 16:30, ddmoorehouseov75lc
Correct relationship between molecular formula and empirical formula
Answers: 1

Chemistry, 23.06.2019 01:00, shartiarahoward
Atoms contain subatomic particles called protons and neutrons. when these protons and neutrons spilt, a lot of energy is released
Answers: 3

Chemistry, 23.06.2019 01:30, yarrito20011307
Which conclusion fits the data in the table? a. heat chemically changes chocolate and margarine. b. all solids become liquid at 100°f. c. removing heat from a substance it to melt. d. matter may change shape when it is heated.
Answers: 1
You know the right answer?
The Lewis dot model of a molecule is shown. Based on the model, which of the following is true? (5 p...
Questions in other subjects:

Physics, 31.01.2020 22:51

Biology, 31.01.2020 22:51


Biology, 31.01.2020 22:51


Mathematics, 31.01.2020 22:51

English, 31.01.2020 22:51


English, 31.01.2020 22:51

History, 31.01.2020 22:51



