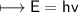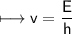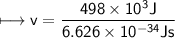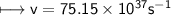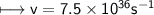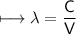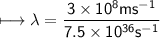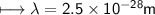Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:00, lydiadmanautou04
Write a hypothesis that answers the lesson question, “while observing a chemical reaction, how can you tell which reactant is limiting? ” hypothesis: if a substance is the limiting reactant, then . . because . .
Answers: 1

Chemistry, 22.06.2019 15:20, Tringirl233
Identify arrows pointing to bonding electrons. done h-0-0-h ) intro
Answers: 3

Chemistry, 22.06.2019 23:00, lulprettyb
What is the most common reason for matter changing its state?
Answers: 1

Chemistry, 23.06.2019 09:20, taylorannsalazar
La reaccion entre monoxido de nitrogeno (no) y oxigeno para formardioxido de nitrogeno (no2) es un paso determinante para la formacion del smog, la reaccion es la siguiente: 2no + o2 = 2no2 cual sera el numero de moles de no2 que se formaran por la reaccion completa de 8 moles de oxigeno con suficiente monoxido?
Answers: 1
You know the right answer?
It takes 498. kJ/mol to break an oxygen-oxygen double bond. Calculate the maximum wavelength of ligh...
Questions in other subjects:

Mathematics, 04.03.2021 21:50


Mathematics, 04.03.2021 21:50

Mathematics, 04.03.2021 21:50





Spanish, 04.03.2021 21:50

Mathematics, 04.03.2021 21:50