
Chemistry, 11.09.2021 03:10 fordenboer
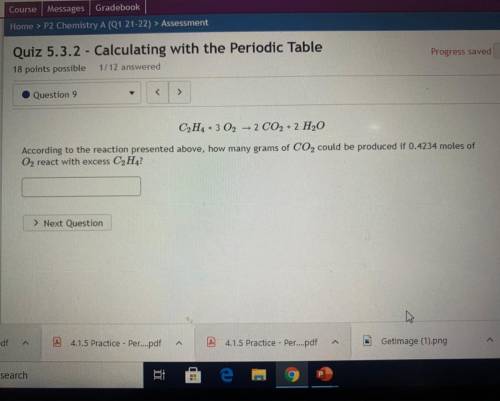
C2H4 + 3 02 – 2 CO2 + 2 H2O
According to the reaction presented above, how many grams of CO2 could be produced if 0.4234 moles of
O2 react with excess C2H4?
Someone PLEASE HELP

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:30, TheRealKodakBlack
What is a scientific theory? a. a scientist's guess about how something works b. the results of an experiment obtained using the scientific method c. a proven fact that will never change d. an idea that is backed by data from many sources
Answers: 2

Chemistry, 22.06.2019 10:00, halohero7
3. how much energy in joules is required to evaporate .0005 kg of liquid ammonia to vapor at the same temperature? 4. how much energy ( in megajoules ) is given up by .75 kg of water at 0c when it freezes to form ice at 0c? 5. explain how heat works between and at critical temperatures?
Answers: 2


Chemistry, 22.06.2019 17:00, brownvester44
Astable electron arrangement for an atom is one that does not easily change. how is this arrangement arrived at? a. valence electrons are transferred or shared to create a full outer shell of electrons. b. valence electrons are discarded into space to create a full outer shell of electrons. c. protons (positive charge) pair with valence electrons (negative charge) to create a strong bond. d. outer shells with valence electrons are transferred or shared.
Answers: 2
You know the right answer?
C2H4 + 3 02 – 2 CO2 + 2 H2O
According to the reaction presented above, how many grams of CO2 could...
Questions in other subjects:













