
Chemistry, 07.09.2021 19:40 jpatte2oye8qv
The polyatomic nitrate ion (NO3−) is present in both the products and the reactants of the chemical equation shown below.
Ba(NO3)2 + 2LiOH → Ba(OH)2 + 2LiNO3
How many nitrate ions are in the products and the reactants of the chemical equation?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 21:40, fatherbamboo
A5 mole sample of liquid acetone is converted to a gas at 75.0°c. if 628 j are required to raise the temperature of the liquid to the boiling point, 15.600 kj are required to evaporate the liquid, and 712 j are required to raise the final temperature to 75.0°c, what is the total energy required for the conversion?
Answers: 3

Chemistry, 23.06.2019 00:30, hdhshshs741
An unknown insoluble substance displaced the water shown. it's mass is indicated on the triple beam balance. mass = a. 694 b. 693.5 c. 693.0 d.693.8
Answers: 1

You know the right answer?
The polyatomic nitrate ion (NO3−) is present in both the products and the reactants of the chemical...
Questions in other subjects:





Mathematics, 03.11.2020 21:50

Mathematics, 03.11.2020 21:50

Social Studies, 03.11.2020 21:50



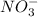 , so we just need to count how many of them are on each side of the equation.
, so we just need to count how many of them are on each side of the equation. , which shows that there are two nitrate ions in the reactant side (as seen by the little 2).
, which shows that there are two nitrate ions in the reactant side (as seen by the little 2).

