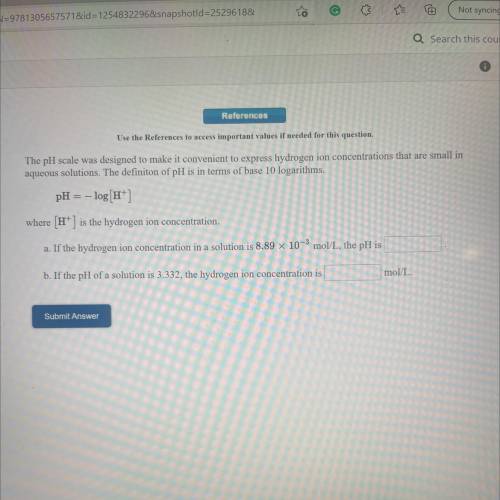
Use the References to access important values if needed for this question.
H
A-Z
The p...

Chemistry, 06.09.2021 05:50 destiny465
Use the References to access important values if needed for this question.
H
A-Z
The pH scale was designed to make it convenient to express hydrogen ion concentrations that are small in
aqueous solutions. The definiton of pH is in terms of base 10 logarithms.
pH = -log[H+]
where (H+) is the hydrogen ion concentration.
a. If the hydrogen ion concentration in a solution is 8.89 x 10-3 mol/L, the pH is
b. If the pH of a solution is 3.332, the hydrogen ion concentration is
mol/L.
Submit Answer

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:00, tamikagoss22
Agas in a balloon at constant pressure has a volume of 160 ml at -125*c. what is its volume at 29.0*c?
Answers: 1

Chemistry, 22.06.2019 00:00, lakenyagillard79
Acurium-245 nucleus is hit with a neutron and changes as shown by the equation. complete the equation by filling in the missing parts. 52
Answers: 2

Chemistry, 22.06.2019 10:40, justicejesusfreak
Which buffer would be better able to hold a steady ph on the addition of strong acid, buffer 1 or buffer 2? explain. buffer 1: a solution containing 0.10 m nh4cl and 1 m nh3. buffer 2: a solution containing 1 m nh4cl and 0.10 m nh3
Answers: 1
You know the right answer?
Questions in other subjects:




English, 10.07.2019 13:10

History, 10.07.2019 13:10


Mathematics, 10.07.2019 13:10





