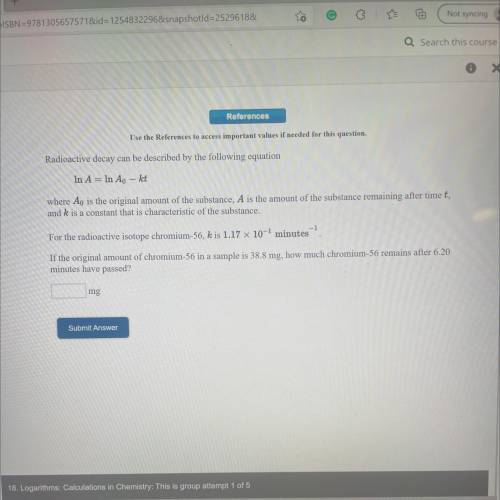
Radioactive decay can be described by the following equation
In A = In Ao – kt
where Ao is t...

Chemistry, 06.09.2021 05:40 TheOriginal2x
Radioactive decay can be described by the following equation
In A = In Ao – kt
where Ao is the original amount of the substance, A is the amount of the substance remaining after timet,
and k is a constant that is characteristic of the substance.
For the radioactive isotope chromium-56, k is 1.17 x 10-2 minutes
If the original amount of chromium-56 in a sample is 38.8 mg, how much chromium-56 remains after 6.20
minutes have passed?
mg

Answers: 2


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 12:10, yootmytoot
Achemistry student needs to standardize a fresh solution of sodium hydroxide. he carefully weighs out of oxalic acid , a diprotic acid that can be purchased inexpensively in high purity, and dissolves it in of distilled water. the student then titrates the oxalic acid solution with his sodium hydroxide solution. when the titration reaches the equivalence point, the student finds he has used of sodium hydroxide solution. calculate the molarity of the student's sodium hydroxide solution. be sure your answer has the correct number of significant digits.
Answers: 1
You know the right answer?
Questions in other subjects:

Mathematics, 14.04.2021 04:10

Mathematics, 14.04.2021 04:10

Mathematics, 14.04.2021 04:10

English, 14.04.2021 04:10




Arts, 14.04.2021 04:10


Mathematics, 14.04.2021 04:10



