
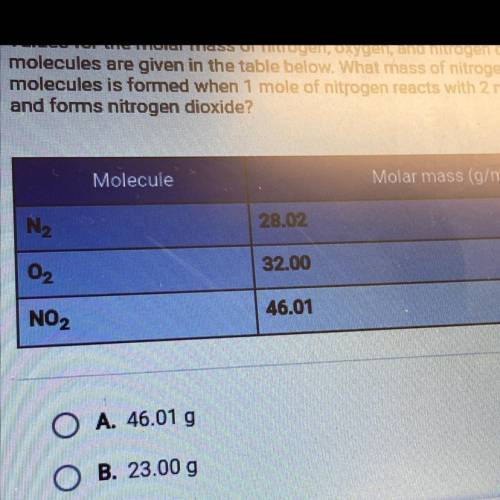
Values for the molar mass of nitrogen, oxygen, and nitrogen dioxide
molecules are given in the table below. What mass of nitrogen dioxide
molecules is formed when 1 mole of nitrogen reacts with 2 moles of oxygen
and forms nitrogen dioxide?
A. 46.01 g
B. 23.00 g
C. 2.00 g
D. 92.02 g

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:00, heids17043
Chen drew a diagram to compare the ways in which different organisms obtain nitrogen. which label belongs to the area marked z?
Answers: 3

Chemistry, 22.06.2019 17:10, mikeeway33
Some liquids can be distilled, but only at temperatures that are so high that it is impractical, or so high the compound decomposes. explain why distillation such compounds at significantly less than atmospheric pressure (some degree of vacuum) would solve this problem.
Answers: 2

Chemistry, 22.06.2019 17:50, kaylamount
Cryolite, na3alf6(s), an ore used in the production of aluminum, can be synthesized using aluminum oxide. start this question by first balance the chemical equation.1.) balance the equation: - alo3(s)+naoh(l)+hf(> na3alf6+h2o(g). 2.) if 17.5 kilograms of al2o3(s), 51.4 kilograms of naoh(l), and 51.4 kilograms of hf(g) react completely, how many kilograms of cryolite will be produced? 3.)which reactants will be in excess, (al2o3, naoh, or hf) 4.)what is the total mass of the excess reactants left over after the reaction is complete in kg?
Answers: 2
You know the right answer?
Values for the molar mass of nitrogen, oxygen, and nitrogen dioxide
molecules are given in the tab...
Questions in other subjects:


Mathematics, 08.09.2019 10:10


Physics, 08.09.2019 10:10

Mathematics, 08.09.2019 10:10

Mathematics, 08.09.2019 10:10

Mathematics, 08.09.2019 10:10

History, 08.09.2019 10:10




