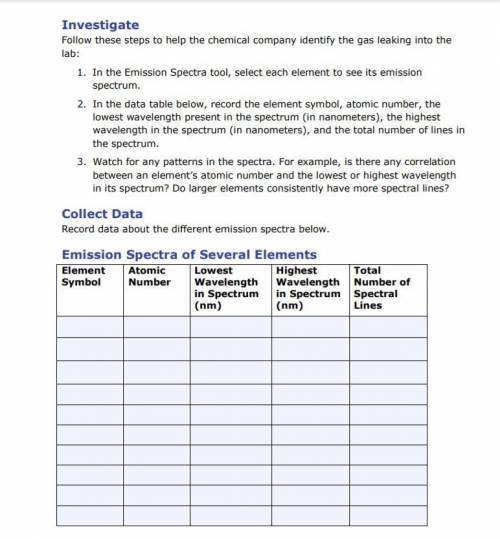
1. In the Emission Spectra tool, select each element to see its emission
spectrum.
2. In th...

1. In the Emission Spectra tool, select each element to see its emission
spectrum.
2. In the data table below, record the element symbol, atomic number, the
lowest wavelength present in the spectrum (in nanometers), the highest
wavelength in the spectrum (in nanometers), and the total number of lines in
the spectrum.
3. Watch for any patterns in the spectra. For example, is there any correlation
between an element’s atomic number and the lowest or highest wavelength
in its spectrum? Do larger elements consistently have more spectral lines?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 14:10, gizmo50245
Calculate the mass percent of hydrogen in methyl acetate
Answers: 1

Chemistry, 22.06.2019 00:10, scottbrandon653
Think about how you can use le chatelier’s principle to find possible solutions to the design problem. describe at least two ways to increase the yield (amount) of ammonia based on this principle.
Answers: 2

Chemistry, 22.06.2019 13:30, richardwalker8ourhg2
An animal cell loses the ability to convert energy stored in food to energy that the cell can use. which of the cell's organelles has stopped working? a. the mitochondria b. the nucleus c. the vacuoles d. the endoplasmic reticulum
Answers: 1
You know the right answer?
Questions in other subjects:

Mathematics, 17.09.2020 04:01

English, 17.09.2020 04:01

French, 17.09.2020 04:01

English, 17.09.2020 04:01

English, 17.09.2020 04:01

Mathematics, 17.09.2020 04:01

Mathematics, 17.09.2020 04:01

German, 17.09.2020 04:01

Mathematics, 17.09.2020 04:01

Mathematics, 17.09.2020 04:01



