
Chemistry, 27.08.2021 06:50 amandasantiago2001
Please help I have 15 min to respond this
1. Magnesium is the limiting reactant in this experiment. Calculate the theoretical yield of MgO for each trial.
2. Determine the percent yield of MgO for your experiment for each trial.
3. Determine the average percent yield of MgO for the two trials.
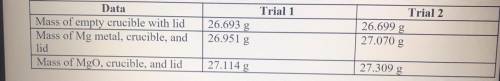

Answers: 3


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 16:10, 00015746
Predict the reactants of this chemical reaction. that is, fill in the left side of the chemical equation. be sure the equation you submit is balanced. (you can edit both sides of the equation to balance it, if you need to.) note: you are writing the molecular, and not the net ionic equation. > cacl2(aq) + h20(l)
Answers: 2

Chemistry, 23.06.2019 01:00, skatelife8974
What is the chemical name of the compound ti2o3?
Answers: 2
You know the right answer?
Please help I have 15 min to respond this
1. Magnesium is the limiting reactant in this experiment...
Questions in other subjects:



Biology, 28.03.2020 22:57

Mathematics, 28.03.2020 22:57






Social Studies, 28.03.2020 22:57



