What is the freezing point, in °C,
of a 1.56 m aqueous solution
of K3PO4?
Note: K for...

Chemistry, 25.08.2021 20:10 kyahshayovvu24
What is the freezing point, in °C,
of a 1.56 m aqueous solution
of K3PO4?
Note: K for water is 1.86 °C/m
Enter rounded answer to three decimal points.
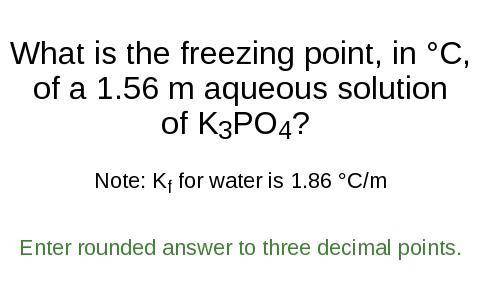

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 13:30, makenziehook8
Which is true of a liquid? it has a definite volume but not a definite mass. it has a definite mass but not a definite volume. it has a definite volume but not a definite shape. it has a definite shape but not a definite volume.
Answers: 2

Chemistry, 23.06.2019 06:00, asalimanoucha2v
•what conclusions can you make about the relationship between the volume of a gas and its temperature? • what conclusions can you make about the relationship between the volume of a gas and its pressure? • what possible variables have you not accounted for? as you did the procedures, is it possible that the atmospheric pressure may have changed? if it did change over the course of your experiment, then how would your results have been affected?
Answers: 3

Chemistry, 23.06.2019 10:00, Jennifer16253
What is the mass in grams of 12.26 ml of acetone
Answers: 1
You know the right answer?
Questions in other subjects:

Social Studies, 06.05.2021 21:20

English, 06.05.2021 21:20

Mathematics, 06.05.2021 21:20



Mathematics, 06.05.2021 21:20



Mathematics, 06.05.2021 21:20

English, 06.05.2021 21:20



