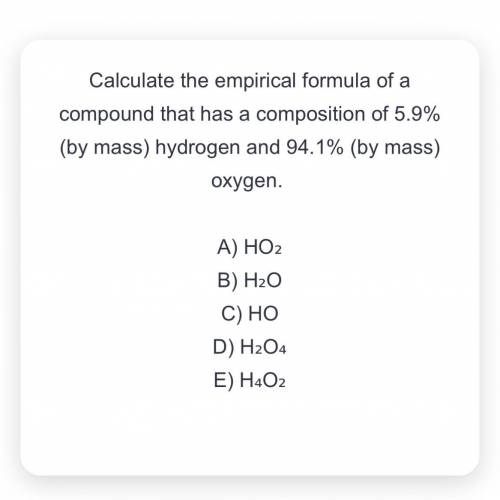
Chemistry, 21.08.2021 02:00 garciaarmando13
Calculate the empirical formula of a compound that has a composition of 5.9% (by mass) hydrogen and 94.1% (by mass) oxygen.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:20, whrjegt4jrnfdvj
1. suppose a reaction mixture, when diluted with water, afforded 300 ml of an aqueous solution of 30 g of the reaction product malononitrile [ch2(cn)2], which is to be isolated by extraction with ether. the solubility of malononitrile in ether at room temperature is 20.0 g/100 ml, and in water is 13.3 g/100 ml. what weight of malononitrile would be recovered by extraction with (a) three 100-ml portions of ether and (b) one 300-ml portion of ether? suggestion: for each extraction, let x equal the weight extracted into the ether layer. in part (a), the concentration in the ether layer is x/100 and in the water layer is (30 x)/300; the ratio of these quantities is equal to k 20/13.3.
Answers: 2

Chemistry, 22.06.2019 06:00, nikejose11
An alkaline battery produces electrical energy according to the following equation. zn(s) + 2 mno2(s) + h2o(l) zn(oh)2(s) + mn2o3(s) (a) determine the limiting reactant if 17.5 g zn and 31.0 g mno2 are used. (type your answer using the format ch4 for ch4.) (b) determine the mass of zn(oh)2 produced. _ g
Answers: 3

Chemistry, 22.06.2019 08:30, myamiller558
Which of the following would be an accurate picture of the earth during the summer time of the northern hemisphere?
Answers: 1
You know the right answer?
Calculate the empirical formula of a compound that has a composition of 5.9% (by mass) hydrogen and...
Questions in other subjects:



Biology, 13.11.2020 23:40

Biology, 13.11.2020 23:40


Spanish, 13.11.2020 23:40

Mathematics, 13.11.2020 23:40



Mathematics, 13.11.2020 23:40