
Chemistry, 20.08.2021 08:20 adaneri1234
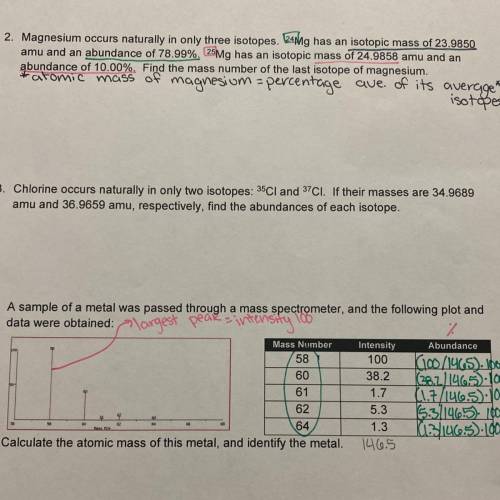
Magnesium occurs naturally in only three isotopes. ^24 Mg has an isotopic mass of 23.9850 amu and an abundance of 78.99%. ^25 Mg has an isotopic mass of 24.9858 amu and an abundance of 10.00%. Find the mass number of the last isotope of magnesium. Can someone please explain how to solve this? *done mine the notes*

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:50, kyleighmarie05
H2so4(aq) + mg(s)—> mgso4(aq) +h2(g) which substance is the acid in the reaction?
Answers: 3

Chemistry, 22.06.2019 05:50, mrylenastewart
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3 -> a2cr(no3)3 + a3ag2so4
Answers: 1

Chemistry, 22.06.2019 07:10, jasondesatnick
An experimental procedure requires a 10 ml of acid to be dissolved
Answers: 2

Chemistry, 22.06.2019 21:00, estherdinhllama
Read "who built the pyramids? ”. leave this link open while you answer the questions throughout the assignment. give at least two reasons why some people claim the pyramids of giza were constructed by aliens.
Answers: 1
You know the right answer?
Magnesium occurs naturally in only three isotopes. ^24 Mg has an isotopic mass of 23.9850 amu and an...
Questions in other subjects:


History, 06.01.2021 23:50

Mathematics, 06.01.2021 23:50


Chemistry, 06.01.2021 23:50


Mathematics, 06.01.2021 23:50



History, 06.01.2021 23:50



