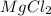Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:30, asianaenaeh
Select the correct answer. when carbon dioxide dissolves in water, it sometimes reacts with water to form carbonic acid as in this balanced equation: co2 + h2o → h2co3. if 495 milliliters of carbon dioxide at 25°c and 101.3 kilopascals reacts with excess water, what is the theoretical yield of carbonic acid? use the periodic table and the ideal gas resource a. 0.889 g b. 1.10g c. 1.27g d. 2.02g what's the answer! quick!
Answers: 1

Chemistry, 22.06.2019 05:00, adjjones2011
As you watch a surfer ride a wave towards the shoreline, what is the shoreline? a) displacement reference b) reference point c) coordinate plane d) cartesian boundary
Answers: 1


Chemistry, 22.06.2019 21:00, andrethisman88
Kp is the equilibrium constant for dissociation of the propionic acid dimer. what is the sign of the slope for a plot of the natural logarithm of kp vs. inverse temperature for this reaction?
Answers: 1
You know the right answer?
Determine the empirical formula of the compound formed when 1.2g of magnesium reacts with 3.55g of c...
Questions in other subjects:

Mathematics, 05.05.2020 23:24

Mathematics, 05.05.2020 23:24

Spanish, 05.05.2020 23:24

History, 05.05.2020 23:24

Mathematics, 05.05.2020 23:24

Mathematics, 05.05.2020 23:24

Mathematics, 05.05.2020 23:24

Mathematics, 05.05.2020 23:24

Mathematics, 05.05.2020 23:24

Mathematics, 05.05.2020 23:24