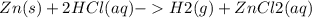A sample of brass weighing 1.203g was analyzed. The zinc in the alloy was reacted with 35.123g of HCl in excess, according to the balanced equation:
Zn (s) + 2HCl(aq) - > H2(g) + ZnCl2 (aq)
After all the zinc reacted, the mass of the remaining solution weighed 36.309g
What was the mass of H2 produced?
What mass of Zn reacted?
What was the percentage of Zn (by mass) in the alloy?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 11:00, justarando
Which element would mostly likely have an electron affinity measuring closest to zero
Answers: 3

Chemistry, 22.06.2019 13:30, amandajbrewerdavis
Table sugar completely dissolved in water is an example of a?
Answers: 1

Chemistry, 22.06.2019 20:00, AaronEarlMerringer
What is the molar mass of the anhydrous compound? answer using four significant figures. 36.02 g/mol 120.15 g/mol 156.12 g/mol
Answers: 1

Chemistry, 22.06.2019 23:00, 1315055427
Which subshell is represented by the actinides family?
Answers: 1
You know the right answer?
A sample of brass weighing 1.203g was analyzed. The zinc in the alloy was reacted with 35.123g of HC...
Questions in other subjects:

World Languages, 16.10.2020 08:01


Mathematics, 16.10.2020 08:01



History, 16.10.2020 08:01

Social Studies, 16.10.2020 08:01

Mathematics, 16.10.2020 08:01

Mathematics, 16.10.2020 08:01

Mathematics, 16.10.2020 08:01


 = 0.0087 moles
= 0.0087 moles