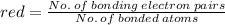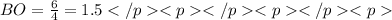Chemistry, 10.08.2021 02:10 vtrvfrfvrvfvnkjrf
The average bond order is the number of bonds between two atoms taking into account resonance.
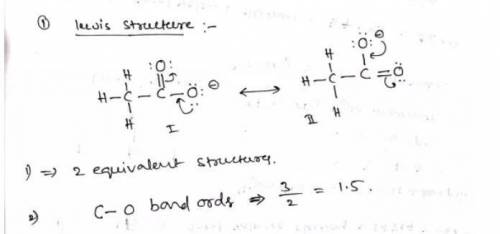
a. Draw a Lewis structure for the nitrite ion and answer the questions below.
1. there are equivalent Lewis structures for NO2-.
2. the average N-O bond order is .
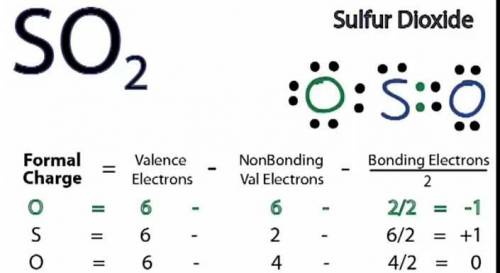
b. Draw a Lewis structure for sulfur dioxide and answer the questions below.
1. there are equivalent Lewis structures for SO2.
2. the average S-O bond order is .

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:30, huangjianhe135
Start an single atom tab. observe the decay of polonium-211. after each decay, press the reset nucleus button to watch the process again. write a description of alpha decay for po-211
Answers: 2

Chemistry, 22.06.2019 04:00, tifftifftiff5069
You encounter a solution that is acidic and you decide to test it by adding a small amount of a strong acid. the ph lowers slightly but is approximately unchanged, and still remains acidic. what can you say about the solution? a. it is a buffer solution. b. it is not a buffer solution it is a strong acid solution. d. the solution has been neutralized. e. the solution has excess acid present
Answers: 1

Chemistry, 22.06.2019 07:00, daniellekennedy05
If there is any 12 to 14 girls that need a boyfriend just follow me and let me know
Answers: 1
You know the right answer?
The average bond order is the number of bonds between two atoms taking into account resonance.
a....
Questions in other subjects:


Chemistry, 21.08.2020 02:01


Mathematics, 21.08.2020 02:01



Mathematics, 21.08.2020 02:01