
Chemistry, 09.08.2021 14:00 ilovecatsomuchlolol
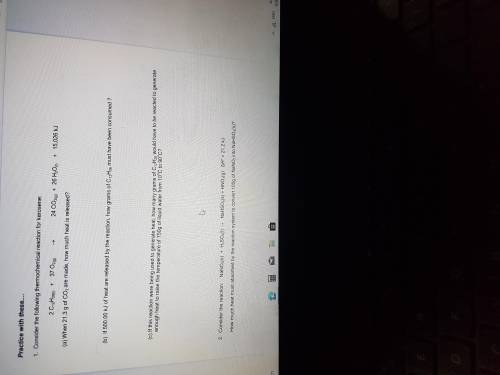
Consider the following thermochemical reaction for kerosene:
2CH12H26(l)+37O2(g).24CO2(g)+26H2O( l)+15,026kj
A. When 21.3g of CO2 are made, how much heat is released ?
B. If 500.00kj of heat are released by the reaction, how many grams of C12H26 must be produced?
C. If this reaction were to being used to generate heat , how many grams of C12H26 would have to be reacted to generate enough heat to raise the temperature of 750g of liquid water from 10 degrees Celsius to 90 degrees celsius ?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:30, alexandroperez13
It takes 945.kj/mol to break a nitrogen-nitrogen triple bond. calculate the maximum wavelength of light for which a nitrogen-nitrogen triple bond could be broken by absorbing a single photon.
Answers: 3


Chemistry, 22.06.2019 04:40, deedee363
*will mark you brainliest + 15 points ** why does the equilibrium of a system shift when the pressure is increased? a. to maximize the stress on the system b. to stop restoring equilibrium to the system c. to increase the total moles of gas in the system d. to decrease the total moles of gas in the system
Answers: 3

Chemistry, 22.06.2019 05:20, jtingley0502
Temperature is _related to the average kinetic energy of a gas. inversely directly not disproportionally
Answers: 1
You know the right answer?
Consider the following thermochemical reaction for kerosene:
2CH12H26(l)+37O2(g).24CO2(g)+26H2O( l...
Questions in other subjects:


Mathematics, 09.07.2019 14:30

Chemistry, 09.07.2019 14:30

Social Studies, 09.07.2019 14:30

Mathematics, 09.07.2019 14:30

Health, 09.07.2019 14:30

Social Studies, 09.07.2019 14:30

Physics, 09.07.2019 14:30

English, 09.07.2019 14:30



