
Chemistry, 08.08.2021 06:10 lillovecandy
Click on Molarity and Density to expand that section. Then scroll to the bottom and click on Alcohol
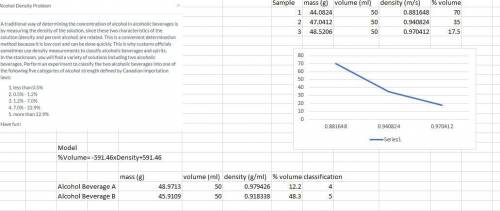
Density Problem.
Everything else you will need is available on the left side of the screen under one of three tabs:
Solutions, Glassware or Tools. Clicking any one of those will give you a list of choices. You might have to
expand a section to see all of the options. When you find what you want, you can click the name of the
item to add it to the workbench, which serves as the main area of the virtual lab. In this experiment you
will choose the size and type of glassware that you use.
When you click on a container on the workbench with a chemical in it, you will see an Information bar
on the left side that provides specific information about that solution or substance. When you have
clicked on a container on the workbench containing a solution, you can control what information is
displayed by clicking on View at the top of the screen. A drop-down will appear with choices that you
can check or uncheck. For this experiment, uncheck Solution Properties.
Conclusion
The conclusion is a double-spaced, 1-paragraph summary (250 -300 words) of the experiment in which
you:
1. Summarize, in one or two sentences, the overall goal of the experiment.
2. Summarize, in one or two sentences, the overall procedure used to accomplish the goals.
3. Present your final results. Note that the results are not the same thing as the data that is
collected. You do not need to present your data in the conclusion.
4. Use your results and observations to comment upon any sources of unavoidable error that
would be present if this experiment were performed in-person. Note: human error is not an
acceptable answer since it is avoidable.
5. Was the calculated concentration of the alcohol reasonable? Completing the calculations
correctly is not an indication of reasonableness.
6. What improvements, if any, could you make to the procedure if you were completing this
experiment in an actual laboratory?
In scientific writing, third-person, passive voice is used. This keeps the focus on the experiment, rather
than experimenter. For example, “A 5-mL volume of solution was poured into an evaporating dish” is
proper. Writing “We poured a 5-mL volume of solution into an evaporating dish,” is improper.

Answers: 2


Other questions on the subject: Chemistry



Chemistry, 23.06.2019 02:30, elyzarobertson
Which statement best describes the liquid state of matter? a. it has definite shape but indefinite volume. b. it has definite shape and definite volume. c. it has indefinite shape and indefinite volume. d. it has indefinite shape but definite volume.
Answers: 1

Chemistry, 23.06.2019 05:00, jayden6467
How many moles are in 7.2 x 10^23 carbon molecules?
Answers: 1
You know the right answer?
Click on Molarity and Density to expand that section. Then scroll to the bottom and click on Alcohol...
Questions in other subjects:

Mathematics, 29.09.2021 17:50



Mathematics, 29.09.2021 17:50


Physics, 29.09.2021 17:50

English, 29.09.2021 17:50



Mathematics, 29.09.2021 17:50



