
Chemistry, 07.08.2021 01:00 radaishasmithoxngbj
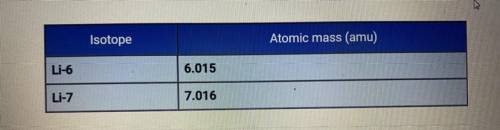
The average atomic mass of lithium is 6.94 amu. Based on the atomic
masses of the two isotopes of lithium, how do the relative abundances of the
isotopes compare?
A. Li-6 is much more abundant than Li-7.
B. They are about the same.
C. Li-7 is much more abundant than Li-6.
D. Li-7 is slightly more abundant than Li-6.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:10, mpchop
According to the diagram; a) identify the anode of the cell and write the half-reaction that occurs there b) write the overall equation for the reaction that occurs as the cell operates c) calculate the value of the standard cell potential ,e cell. d)write the shorthand notation of the cell above e)indicate the flow of the electrons on the diagram
Answers: 3

Chemistry, 22.06.2019 14:30, Dreynolds1667
100 grams of molten lead (600°c) is used to make musket balls. if the lead shot is allowed to cool to room temperature (21°c), what is the change in entropy (in j/k) of the lead? (for the specific heat of molten and solid lead use 1.29 j/g⋅°c; the latent heat of fusion and the melting point of lead are 2.45 × 104 j/kg and 327°c, respectively.)
Answers: 1


Chemistry, 22.06.2019 21:20, skyemichellec
40dm3 of gas at 760 torr are heated from 5°c to 50°c what is the new volume
Answers: 3
You know the right answer?
The average atomic mass of lithium is 6.94 amu. Based on the atomic
masses of the two isotopes of l...
Questions in other subjects:


Mathematics, 01.12.2021 03:10


Business, 01.12.2021 03:10

Mathematics, 01.12.2021 03:10




Social Studies, 01.12.2021 03:10

Physics, 01.12.2021 03:10



