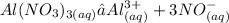Chemistry, 06.08.2021 22:30 kyramillerr8639
Which of the following has the greatest effect on colligative properties?
A. Calcium chloride (CaCl2)
B. Sodium chloride (NaCl)
C. Aluminum Nitrate (Al(NO3)3)
D. Epsom salt (MgSO4)

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 13:00, aleilyg2005
If two objects at different te, peraure are in contact with each other what happens to their temperature
Answers: 1

Chemistry, 22.06.2019 14:30, joejoefofana
Consider the reduction reactions and their equilibrium constants. cu+(aq)+e−↽−−⇀cu(s)pb2+(aq)+2e−↽−−⇀ pb(s)fe3+(aq)+3e−↽−−⇀fe(=6.2×108=4. 0×10−5=9.3×10−3 cu + ( aq ) + e − ↽ − − ⇀ cu ( s ) k =6.2× 10 8 pb 2 + ( aq ) +2 e − ↽ − − ⇀ pb ( s ) k =4.0× 10 − 5 fe 3 + ( aq ) +3 e − ↽ − − ⇀ fe ( s ) k =9.3× 10 − 3 arrange these ions from strongest to weakest oxidizing agent.
Answers: 3

Chemistry, 22.06.2019 14:50, wcraig1998
Complete the following statements to describe solids, liquids, and gases. select the correct answer from each drop-down menu. a solid a definite volume and a definite shape. a liquid a definite volume and a definite shape. a gas a definite volume and a definite shape
Answers: 1

Chemistry, 22.06.2019 16:00, yfnal3x
What rule is used to determine how many covalent bonds an element can form? a. the number of covalent bonds is equal to six c the number of covalent bonds is equal to five minus the group number plus the group number b. the number of covalent bonds is equal to eight d. none of the above minus the group number select the best answer from the choices provided
Answers: 2
You know the right answer?
Which of the following has the greatest effect on colligative properties?
A. Calcium chloride (CaCl...
Questions in other subjects:




Mathematics, 28.07.2019 04:34

Advanced Placement (AP), 28.07.2019 04:34

Mathematics, 28.07.2019 04:34

Mathematics, 28.07.2019 04:34



History, 28.07.2019 04:34