
Chemistry, 06.08.2021 04:40 rogersdeloris1ovgm3b
Acids and Bases Titration
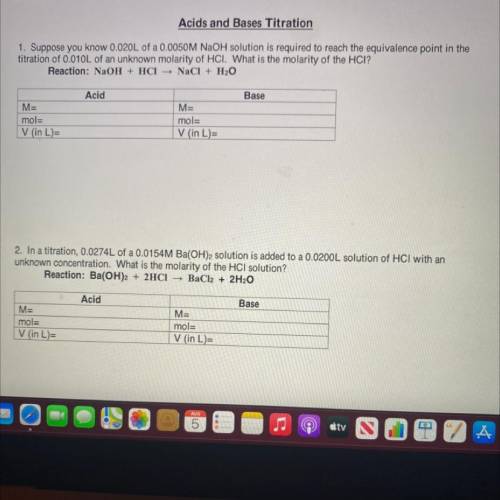
1. Suppose you know 0.020L of a 0.0050M NaOH solution is required to reach the equivalence point in the
titration of 0.010L of an unknown molarity of HCI. What is the molarity of the HCI?
Reaction: NaOH + HCl NaCl + H2O
Acid
Base
M=
mol=
V (in L)=
M=
mol=
V (in L)=

Answers: 2


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 10:00, paynedeforest2596
According to the tide table below what time of day will the highest tide occur? (2 pt) the highest tide will occur at
Answers: 1

Chemistry, 22.06.2019 12:00, shifaxoxoxo
What term is applied to a scientist who studies ancient life, including animal and plant fossils a. anthropologist b. dendroclimatologist c. geophysicist d. paleontologist
Answers: 2
You know the right answer?
Acids and Bases Titration
1. Suppose you know 0.020L of a 0.0050M NaOH solution is required to reac...
Questions in other subjects:












