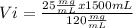Chemistry, 04.08.2021 17:00 wdgyvwyv8840
A stock solution of magnesium chloride has a concentration of 120 mg mL. How many milliliters of the stock solution are required to prepare 1.5 L of 25 mg mL solution

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 15:20, merrickrittany
An alloy contains 66 g of pure zinc. what is the percentage of zinc in the alloy? express your answer to two significant figures and include the appropriate units.
Answers: 1

Chemistry, 22.06.2019 15:30, lovebaeforlife351
Each of the following reactions is allowed to come to equilibrium and then the volume is changed as indicated. predict the effect (shift right, shift left, or no effect) of the indicated volume change. drag the appropriate items to their respective bins. co(g) + h2o(g) < => co2(g) + h2(g) (volume is decreased) pcl3(g) + cl2(g) < => pcl5(g) (volume is increased) caco3(s)< => cao(s) + co2(g) (volume is increased)
Answers: 1

Chemistry, 22.06.2019 23:30, ninilizovtskt
If maltose undergoes hydrolysis what subunits does it results to?
Answers: 2
You know the right answer?
A stock solution of magnesium chloride has a concentration of 120 mg mL. How many milliliters of the...
Questions in other subjects:

English, 25.03.2020 05:32



Chemistry, 25.03.2020 05:33


Biology, 25.03.2020 05:33


Physics, 25.03.2020 05:33

History, 25.03.2020 05:33

Mathematics, 25.03.2020 05:33

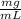 Vi= ?Cf= 1.5 L= 1500 mL (beign 1 L= 1000 mL)Vf= 25
Vi= ?Cf= 1.5 L= 1500 mL (beign 1 L= 1000 mL)Vf= 25