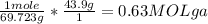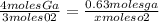Chemistry, 01.08.2021 09:40 keripressley148
4 pts
Question 5
Directions:
Solve the following problem on a separate sheet of paper.
Submit a photo (or scan) to receive credit. File type must be .pdf.
Problem:
Consider the balanced reaction: 4Ga(s) + 302(g) → 2Ga2O3(s)
When gallium metal reacts with 13.0 g of oxygen gas how many grams of gallium oxide will be
produced?
Upload
Choose a File

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 23.06.2019 01:30, jonmorton159
At a certain temperature the rate of this reaction is first order in hi with a rate constant of : 0.0632s2hig=h2g+i2g suppose a vessel contains hi at a concentration of 1.28m . calculate how long it takes for the concentration of hi to decrease to 17.0% of its initial value. you may assume no other reaction is important. round your answer to 2 significant digits.
Answers: 1

Chemistry, 23.06.2019 03:00, kdcloyd3362
Give a real-world example of an energy transformation that uses two of the following forms of energy: chemical, mechanical, nuclear, gravitational, radiant, electrical, thermal (heat), and/or sound.
Answers: 3
You know the right answer?
4 pts
Question 5
Directions:
Solve the following problem on a separate sheet of paper.<...
Directions:
Solve the following problem on a separate sheet of paper.<...
Questions in other subjects:

Mathematics, 05.05.2021 20:20

English, 05.05.2021 20:20

Chemistry, 05.05.2021 20:20





Mathematics, 05.05.2021 20:20