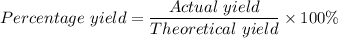Calcium oxide reacts with water in a combination reaction to produce calcium hydroxide: CaO (s) + H2O (l) → Ca(OH)2 (s) In a particular experiment, a 2.00-g sample of CaO is reacted with excess water and 2.14 g of Ca(OH)2 is recovered. What is the percent yield in this experiment? a. 107 b. 1.07 c. 2.88 d. 81.1 e. 93.3

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 16:30, Kathryn014
4. a 20-kg child is tossed up into the air by her parent. the child is 2 meters off the ground traveling 5 m/s. circle one: ke / gpe / both show your work for finding the values of each type of energy the object has:
Answers: 1



Chemistry, 23.06.2019 14:30, ajahbraun
Recognizing the properties of water water has a "bent" geometry. which explanation does not explain why? o water's oxygen has unbonded electron pairs that repel each other. water can form hydrogen bonds. electrons are evenly distributed in the water molecule. do ne
Answers: 3
You know the right answer?
Calcium oxide reacts with water in a combination reaction to produce calcium hydroxide: CaO (s) + H2...
Questions in other subjects:



Biology, 07.12.2020 07:10



World Languages, 07.12.2020 07:10

Biology, 07.12.2020 07:10


Mathematics, 07.12.2020 07:10

Mathematics, 07.12.2020 07:10