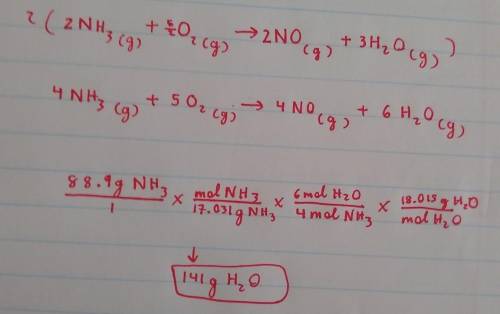Chemistry, 30.07.2021 19:30 cyndalulu6729
If you reacted 88.9 g of ammonia with excess oxygen, what mass of water would you expect to make? You will need to balance the equation first. NH3(g) + O2(g) -> NO(g) + H2O(g)

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:30, fvmousdiana
In france, grapes are 1.95 euros per kilogram. what is the cost of grapes, in dollars per pound, if the exchange rate is 1.14 dollars/euro? (2.6)
Answers: 3

Chemistry, 23.06.2019 01:20, michellectucker1982
Use the de broglie's wave equation to find the wavelength of an electron moving at 7.3 × 106 m/s. show your work. note: h = plank's constant (6.62607 x 10-34 j s)
Answers: 1

Chemistry, 23.06.2019 03:30, Ramann03
27 drag each label to the correct location on the image. a particular exosolar system has five planets in total: a, b, c, d, and e. the table lists the orbital periods of these planets in days. planet orbital period (days) a 600 b 80 c 1,000 d 500 e 100 move each planet to its orbit in the system.
Answers: 3

Chemistry, 23.06.2019 03:50, mobslayer88
How many moles of potassium are needed to react completely with 12.8 moles of magnessium bromide?
Answers: 2
You know the right answer?
If you reacted 88.9 g of ammonia with excess oxygen, what mass of water would you expect to make? Yo...
Questions in other subjects:

Physics, 30.06.2019 15:30


History, 30.06.2019 15:30