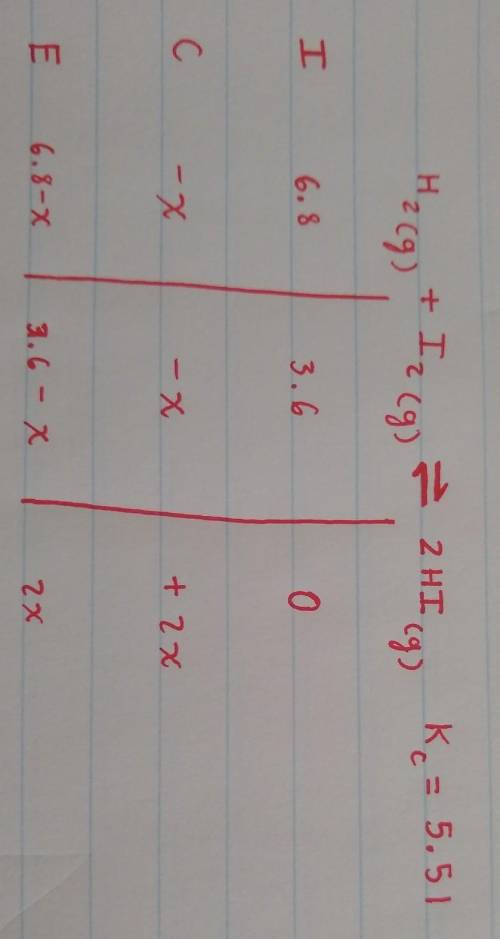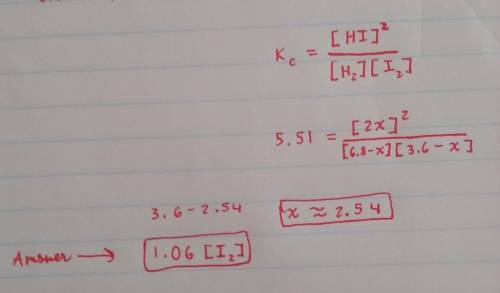Chemistry, 29.07.2021 20:30 vaneayala3078
Suppose a 250.mL flask is filled with 1.7mol of H2 and 0.90mol of I2. The following reaction becomes possible:
+H2gI2g 2HIg
The equilibrium constant K for this reaction is 5.51 at the temperature of the flask.
Calculate the equilibrium molarity of I2. Round your answer to two decimal places.

Answers: 1


Other questions on the subject: Chemistry



Chemistry, 23.06.2019 03:30, elijahjacksonrp6z2o7
In general metals get as you move from left to right across the periodic table.
Answers: 1

You know the right answer?
Suppose a 250.mL flask is filled with 1.7mol of H2 and 0.90mol of I2. The following reaction becomes...
Questions in other subjects:



Biology, 21.01.2021 07:00

Mathematics, 21.01.2021 07:00


English, 21.01.2021 07:00

Biology, 21.01.2021 07:00


English, 21.01.2021 07:00