Chemistry, 28.07.2021 06:30 breannabryan1017
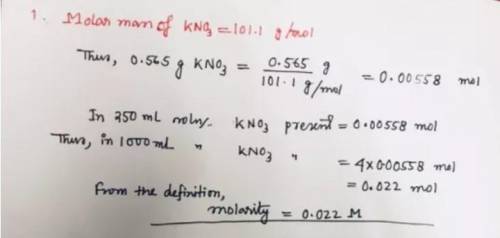
A solution is made by dissolving 0.565 g of potassium nitrate in enough water to make up 250. mL of solution. What is the molarity of this solution? Please explain and show work.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:30, coreyslotte
Use examples from the article to explain one positive and one negative effect that chemistry has had on society.
Answers: 2

Chemistry, 22.06.2019 10:00, aschool2000
Water's surface tension and heat storage capacity are accounted for by its a) orbitals b) weight c) hydrogen bonds d) mass e) size
Answers: 2

Chemistry, 22.06.2019 12:00, carvajalj2520
Explain what happens at the saturation point when adding salt to water at room temperature.
Answers: 1

Chemistry, 23.06.2019 01:00, shartiarahoward
Atoms contain subatomic particles called protons and neutrons. when these protons and neutrons spilt, a lot of energy is released
Answers: 3
You know the right answer?
A solution is made by dissolving 0.565 g of potassium nitrate in enough water to make up 250. mL of...
Questions in other subjects:

Biology, 10.11.2020 01:30

English, 10.11.2020 01:30

Geography, 10.11.2020 01:30

Business, 10.11.2020 01:30



Mathematics, 10.11.2020 01:30