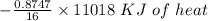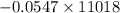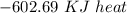Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:30, eburnhisel2023
The volume of helium in a blimp is 6.28 x 10^9 millimeters. the density of helium in the blimp is .1786 kilogram/meter^3. find the mass of the helium in the blimp.
Answers: 1


Chemistry, 22.06.2019 18:30, robjaykay
The famous scientist galileo galilei did several experiments with sloping planes, which he rolled metal balls down so that he could study motion. by changing the slope, he could study how the speed at which the ball rolled was affected. what was the independent variable in galileo's experiment? a. the speed of the ball b. the slope of the plane c. whether the ball moved d. what the ball was made of
Answers: 2

Chemistry, 23.06.2019 01:00, bsheepicornozj0gc
Reactions in cells take place at about a. 40°c b. 0° c. 100°c d. 60°c
Answers: 1
You know the right answer?
Using the following equation for the combustion of octane calculate the heat associated with the for...
Questions in other subjects:


History, 16.02.2021 22:10

Mathematics, 16.02.2021 22:10


Chemistry, 16.02.2021 22:20

Biology, 16.02.2021 22:20

Social Studies, 16.02.2021 22:20



Social Studies, 16.02.2021 22:20

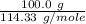
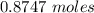
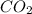 formation associates with -11018 kJ of heat, then
formation associates with -11018 kJ of heat, then