
Chemistry, 27.07.2021 06:30 augestfaith
How many milliliters of 0.204 Mol KMnO4 are needed to react with 3.24 g of iron(II) sulfate, FeSO4? The reation is as folows. 10FeSO4(aq) + 2 KMnO4(aq) = 5Fe2(SO4)3(aq) + 2MnSO4(aq) + K2SO4(aq) + 8H2O(l)
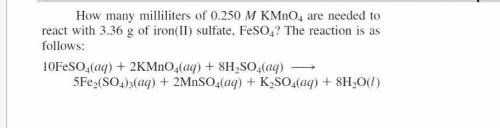

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:50, Catracho3619
Blank allows you to do calculations for situations in which only the amount of gas is constant a)boyle's law b)combined gas law c)ideal gas law d)dalton's law
Answers: 1

Chemistry, 22.06.2019 01:20, sarinaneedshelp01
Match the acid base pairs by arranging the acid name with the conjugate base formula. hydrogen carbonate hydrogen phosphate carbonic acid read water sulfuric acid phosphoric acid a. co32- b. hso4- c. hco3- d. po43- e. h2po4- f. oh-
Answers: 1

Chemistry, 22.06.2019 16:30, Kathryn014
4. a 20-kg child is tossed up into the air by her parent. the child is 2 meters off the ground traveling 5 m/s. circle one: ke / gpe / both show your work for finding the values of each type of energy the object has:
Answers: 1

Chemistry, 22.06.2019 18:40, johnnysteeler9934
What is one real world example of a colligative property?
Answers: 2
You know the right answer?
How many milliliters of 0.204 Mol KMnO4 are needed to react with 3.24 g of iron(II) sulfate, FeSO4?...
Questions in other subjects:


Mathematics, 08.04.2021 14:00


Mathematics, 08.04.2021 14:00

Social Studies, 08.04.2021 14:00

Mathematics, 08.04.2021 14:00



Spanish, 08.04.2021 14:00

History, 08.04.2021 14:00



