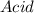In the following acid-base reaction,
H3O+ is the .
HCI(g) + H2O(l) +H30+ (aq) + Cl-(aq)
...

Chemistry, 27.07.2021 03:50 Irishstoner5608
In the following acid-base reaction,
H3O+ is the .
HCI(g) + H2O(l) +H30+ (aq) + Cl-(aq)
A acid
B conjugate acid
C conjugate base

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:30, nekathadon
The bond energy for the van der waals bond between two helium atoms is 7.9×10−4ev. assuming that the average kinetic energy of a helium atom is (3/2)kbt, at what temperature is the average kinetic energy equal to the bond energy between two helium atoms
Answers: 1

You know the right answer?
Questions in other subjects:


English, 23.01.2021 01:20




Mathematics, 23.01.2021 01:20


SAT, 23.01.2021 01:20