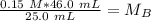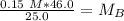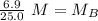Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:00, adrian128383
What forms when chemical reactions combine pollution with sunlight?
Answers: 1

Chemistry, 22.06.2019 09:00, valeriekbueno
If you chip a tooth, most likely you will go to the dentist to have the missing material filled in. currently the material used to fill in teeth is a polymer that is flexible when put in, yet is hardened to the strength of a tooth after irradiation with blue light at a wavelength of 461 nm. what is the energy in joules for a photon of light at this wavelength?
Answers: 1

Chemistry, 22.06.2019 17:10, mikeeway33
Some liquids can be distilled, but only at temperatures that are so high that it is impractical, or so high the compound decomposes. explain why distillation such compounds at significantly less than atmospheric pressure (some degree of vacuum) would solve this problem.
Answers: 2

Chemistry, 23.06.2019 05:00, mprjug6
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 4.20 mol fe and 6.70 mol nio(oh) react?
Answers: 3
You know the right answer?
A titration is performed on a 25.0 mL sample of calcium hydroxide. A volume of
46.0 mL of a 0.15 M...
Questions in other subjects:



Biology, 17.10.2019 04:30

Mathematics, 17.10.2019 04:30

Mathematics, 17.10.2019 04:30




Mathematics, 17.10.2019 04:30

History, 17.10.2019 04:30





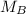 . It is being multiplied by 25.0 milliliters. The inverse operation of multiplication is division, so we divide both sides of the equation by 25.0 mL.
. It is being multiplied by 25.0 milliliters. The inverse operation of multiplication is division, so we divide both sides of the equation by 25.0 mL.