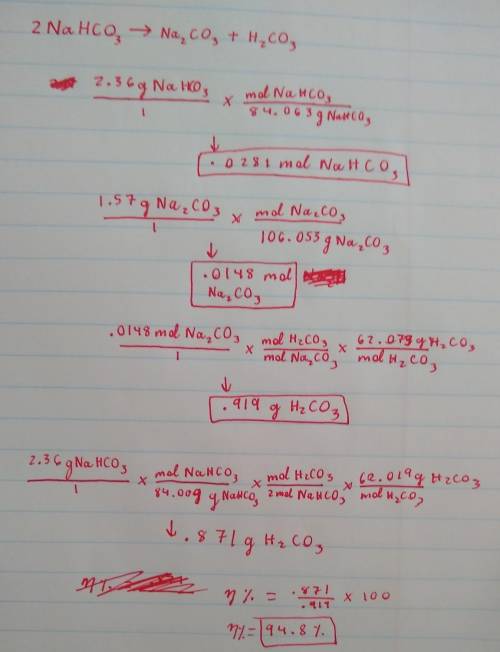ASAP
A 2.36-gram sample of NaHCO3 was completely decomposed in an experiment.
2NaHCO→ Na...

Chemistry, 24.07.2021 01:00 crookdamian21
ASAP
A 2.36-gram sample of NaHCO3 was completely decomposed in an experiment.
2NaHCO→ Na2CO3 + H2CO3
In this experiment, carbon dioxide and water vapors combine to form H2CO3. After decomposition, the Na2CO3 had a mass of 1.57 grams.
Determine the mass of the H2CO3 produced.
Calculate the percentage yield of H2CO3 for the reaction. Show your work or describe the calculation process in detail.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, Cnolteb5663
Often on a topographic map, every fifth contour line is darkened. what is this line called? a. key b. slope c. benchmark d. index contour
Answers: 1

Chemistry, 22.06.2019 07:30, kimberlyrios12p0ts98
Which of the following best supports the concept that genetic information is passed on to offspring from both of their parents, not just one?
Answers: 2


Chemistry, 22.06.2019 12:00, hannah2757
Why are people not able to skip a dive to the deepest part of the ocean
Answers: 1
You know the right answer?
Questions in other subjects:


Mathematics, 02.04.2021 15:00

Social Studies, 02.04.2021 15:00

Physics, 02.04.2021 15:00


Mathematics, 02.04.2021 15:00

Social Studies, 02.04.2021 15:00


Mathematics, 02.04.2021 15:00