
Chemistry, 21.07.2021 21:20 denisebaslee15
What direction would equilibrium moves towards based on the following if we increased the volume of the container.
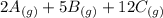 ↔
↔ 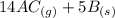
Answer choices:
a) reactants
b) no change
c) products
d) decrease in volume
Please help!

Answers: 3


Other questions on the subject: Chemistry




You know the right answer?
What direction would equilibrium moves towards based on the following if we increased the volume of...
Questions in other subjects:






Mathematics, 21.01.2020 06:31


Mathematics, 21.01.2020 06:31


Mathematics, 21.01.2020 06:31



