
Chemistry, 21.07.2021 15:10 manuellopez1981
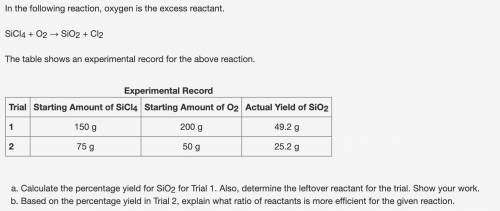
Calculate the percentage yield for SiO2 for Trial 1. Also, determine the leftover reactant for the trial. Show your work. Based on the percentage yield in Trial 2, explain what ratio of reactants is more efficient for the given reaction.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:30, kkelley9223
How would the number of moles (n) of o2 change if the atmospheric pressure doubled but all other variables stayed the same
Answers: 2

Chemistry, 22.06.2019 17:00, brownvester44
Astable electron arrangement for an atom is one that does not easily change. how is this arrangement arrived at? a. valence electrons are transferred or shared to create a full outer shell of electrons. b. valence electrons are discarded into space to create a full outer shell of electrons. c. protons (positive charge) pair with valence electrons (negative charge) to create a strong bond. d. outer shells with valence electrons are transferred or shared.
Answers: 2


Chemistry, 22.06.2019 19:50, ellycleland16
Identify the lewis base in this balanced equation: fe3+ h2o fe(h2o)63+
Answers: 1
You know the right answer?
Calculate the percentage yield for SiO2 for Trial 1. Also, determine the leftover reactant for the t...
Questions in other subjects:

Mathematics, 10.09.2020 08:01

Mathematics, 10.09.2020 08:01

Mathematics, 10.09.2020 08:01

Health, 10.09.2020 08:01

Mathematics, 10.09.2020 08:01

Mathematics, 10.09.2020 08:01

Mathematics, 10.09.2020 08:01

Mathematics, 10.09.2020 08:01

Mathematics, 10.09.2020 08:01

Mathematics, 10.09.2020 08:01



