Chemistry, 19.07.2021 18:20 4presidents
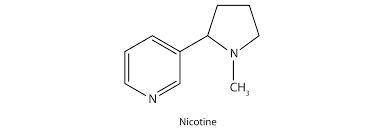
Nicotine is a toxic substance present in tobacco leaves. There are two lone pairs in the structure of nicotine. In general, localized lone pairs are much more reactive than delocalized lone pairs. With this information in mind, do you expect both lone pairs in nicotine to be reactive?
A. Both lone pairs are delocalized and, therefore, both are expected to have the same reactivity.
B. Lone pair in pyrrolidine ring is localized and, therefore, is expected to be more reactive.
C. Both lone pairs are localized and, therefore, both are expected to be reactive.
D. Lone pair in pyridine ring is localized and, therefore, is expected to be more reactive.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:30, murtaghliam1
Word equation for k(s) +h2o(l) yield koh (aq) + h2
Answers: 3

Chemistry, 22.06.2019 22:30, angelagonzalesownus1
Which statement best summarizes the importance of ernest rutherford’s gold foil experiment? it proved that all of john dalton’s postulates were true. it verified j. j. thomson’s work on the atomic structure. it showed that an electron circles a nucleus in a fixed-energy orbit. it showed that a nucleus occupies a small part of the whole atom.
Answers: 1

Chemistry, 23.06.2019 00:00, alisonsolis155
Before it was launched, a helium-filled balloon had a pressure of 201 kpa at a temperature of 27°c. at an altitude of 15,000 m, the pressure had decreased to 2.5 kpa and the temperature had dropped to -14 °c. the volume of the balloon increased to 59.3 m3. what is the original volume of the balloon? 13 m3 0.85 m3 0.077 m3 1.17 m3
Answers: 3

Chemistry, 23.06.2019 03:00, mullerma
Air pressure is measured in pascals. for a professional american football game, the ball should be inflated to about 90,000 pascals. scientists studied the effects of air temperature on the pressure inside american footballs by taking these steps: 1. prepare 100 footballs. 2. measure each football's air pressure. 3. divide footballs into 10 groups. 4. place the groups in different lockers cooled to different air temperatures. 5. after 12 hours, remove the footballs from lockers. 6. measure each football's pressure again. 7. compare the new pressures to the starting pressures. what two terms best describe the variable "air pressure inside the football" in this experiment? independent, qualitative independent, quantitative dependent, qualitative dependent, quantitative
Answers: 3
You know the right answer?
Nicotine is a toxic substance present in tobacco leaves. There are two lone pairs in the structure o...
Questions in other subjects: