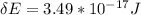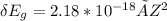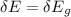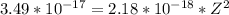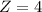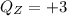Chemistry, 16.07.2021 01:00 theangelsanchez
A one electron species, X m, where m is the charge of the one electron species and X is the element symbol, loses its one electron from its ground state when it absorbs 3.49 x 10-17 J of energy. Using the prior information, the charge of the one electron species is:
a. +8
b. +2
c. +3
d. +1
e. +4

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:30, tae8002001
How much energy moves onto the next level, in an energy pyramid
Answers: 1

Chemistry, 22.06.2019 04:30, EinsteinBro
Which statement best describes the relationship between period and frequency of light waves? a) in wave b the period increases and the frequency decreases from wave a. b) in wave a the period increases and the frequency decreases from wave b. c) in wave b the period is shorter and the frequency is greater than in wave a. d) in wave a the period is shorter and the frequency is greater than in wave b.
Answers: 1

Chemistry, 22.06.2019 13:30, suemmimonjaras8374
The atomic number, or number, is the described as the number of in the nucleus of an chemical element.
Answers: 1

Chemistry, 22.06.2019 14:00, ashlynneboogs0056
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3
You know the right answer?
A one electron species, X m, where m is the charge of the one electron species and X is the element...
Questions in other subjects:

Physics, 18.10.2021 14:00

English, 18.10.2021 14:00

English, 18.10.2021 14:00

Mathematics, 18.10.2021 14:00

Biology, 18.10.2021 14:00


Mathematics, 18.10.2021 14:00

Chemistry, 18.10.2021 14:00

Social Studies, 18.10.2021 14:00

English, 18.10.2021 14:00