
Chemistry, 15.07.2021 21:50 avahrhey24
In aqueous solution the Ni2" ion forms a complex with four ammonia molecules. Write the formation constant expression for the equilibrium between the hydrated metal ion and the aqueous complex. Under that, write the balanced chemical equation for the first step in the formation of the complex K,=.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:30, ElizabethF
Calculate the change in entropy if br2(l) is converted to br2(g). s° for br2(l) = 152.2 j/(mol•k) s° for br2(g) = 245.5 j/(mol•k) s° for br(g) = 175.0 j/(mol•k)
Answers: 3

Chemistry, 22.06.2019 02:30, sotoamerica0814
98 ! and brainliest plz ! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1


Chemistry, 22.06.2019 05:50, mrylenastewart
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3 -> a2cr(no3)3 + a3ag2so4
Answers: 1
You know the right answer?
In aqueous solution the Ni2" ion forms a complex with four ammonia molecules. Write the formation co...
Questions in other subjects:

Chemistry, 21.09.2021 14:00

Physics, 21.09.2021 14:00

Mathematics, 21.09.2021 14:00

Biology, 21.09.2021 14:00

Mathematics, 21.09.2021 14:00

English, 21.09.2021 14:00


Mathematics, 21.09.2021 14:00

Geography, 21.09.2021 14:00

Mathematics, 21.09.2021 14:00

![\frac{[Ni(H_2O)_3 (NH_3)]^{2+}}{[Ni(H_2O)_4]^{2+} [NH_3]}](/tpl/images/1394/7943/a809a.png) ".
".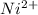 exist as
exist as ![[Ni(H_2O)_4]^{2+}](/tpl/images/1394/7943/49a05.png)
![[Ni(H_2O)_4]^{2+} + 4NH_3 \rightleftharpoons [Ni(NH_3)_4]^{2+} + H_2O](/tpl/images/1394/7943/ab9b4.png)
![K_f = \frac{[Ni(NH_3)_4]^{2+}}{[Ni(H_2O)_4^{2+}] [NH_3]^4}](/tpl/images/1394/7943/d666b.png)
![[H_2O]](/tpl/images/1394/7943/b5d70.png) as concentration of water will be const.
as concentration of water will be const.![[Ni(NH_3)_4]^{2+}](/tpl/images/1394/7943/dff73.png) proceeds via several steps,
proceeds via several steps,![[Ni(H_2O)_4]^{2+}+NH_3 \rightleftharpoons [Ni(H_2O)_3 (NH_3)]^{2+} + H_2O](/tpl/images/1394/7943/b2d29.png)
![K_1 = \frac{[Ni(H_2O)_3 (NH_3)]^{2+}}{[Ni(H_2O)_4]^{2+} [NH_3]}](/tpl/images/1394/7943/01675.png)


