
Chemistry, 15.07.2021 07:20 pineapplepizaaaaa
The base dissociation constant , Kb, of CH3NH2 is 4.4 x 10-4. What does the Kb value indicate about this compound?
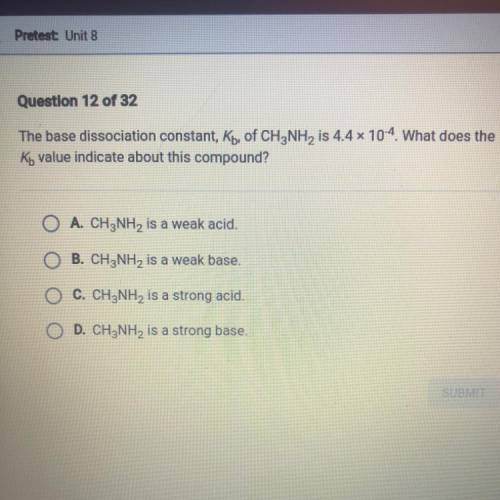

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:00, angtrevv
In a laboratory, 1.55mg of an organic compound containing carbon, hydrogen, and oxygen is burned for analysis. this combustion resulted in the formation of 1.45mg of carbon dioxide and .89 mg of water. what is the empirical formula for this compound?
Answers: 1

Chemistry, 22.06.2019 13:30, annanikherrera
If the concentration of phosphate in the cytosol is 2.0 mm and the concentration of phosphate in the surrounding fluid is 0.1 mm, how could the cell increase the concentration of phosphate in the cytosol? a) passive transportb) diffusionc) active transportd) osmosise) facilitated diffusion
Answers: 3

Chemistry, 23.06.2019 11:50, vmosley8648
It takes 155. kj/mol to break a fluorine-fluorine single bond. calculate the maximum wavelength of light for which a flouine-flouring single bond could be broken by absorbing a single photon
Answers: 1
You know the right answer?
The base dissociation constant , Kb, of CH3NH2 is 4.4 x 10-4. What does the Kb value indicate about...
Questions in other subjects:

Chemistry, 11.10.2019 00:30



Business, 11.10.2019 00:30

Mathematics, 11.10.2019 00:30




Mathematics, 11.10.2019 00:30




