
Chemistry, 15.07.2021 01:30 madisonnxo
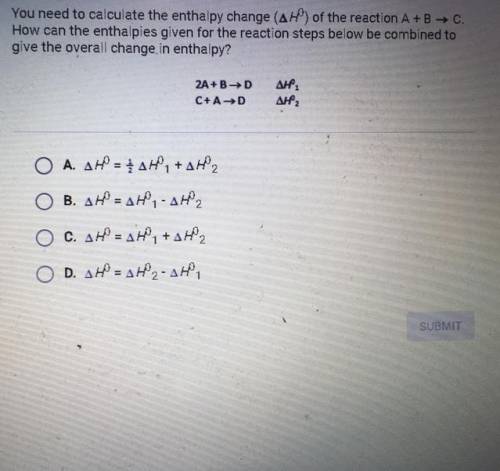
You need to calculate the enthalpy change (∆H0) of the reaction A + B -> C. How can the enthalpies given for the reaction steps below be combined to give the overall change in enthalpy?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:30, greekfreekisdbz
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 3


Chemistry, 22.06.2019 13:10, bartonamber4042
Select the correct answer a modure consists of glucose and water. what is the percent composition of glucose in the mixture if it contains 1.3 moles of glucose (cho total mass of the mature is 276 grams? ) and the a 1775
Answers: 1

Chemistry, 22.06.2019 20:00, bbyjean9974
State one important difference between a physical change and a chemical change?
Answers: 1
You know the right answer?
You need to calculate the enthalpy change (∆H0) of the reaction A + B -> C. How can the enthalpie...
Questions in other subjects:

Mathematics, 28.09.2019 18:30

Mathematics, 28.09.2019 18:30



Social Studies, 28.09.2019 18:30


History, 28.09.2019 18:30



History, 28.09.2019 18:30



