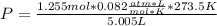Chemistry, 14.07.2021 01:00 LanaParrilla
calculate pressure exerted by 1.255 mol of CI2 in a volume of 5.005 L at a temperature 273.5 k using ideal gas equation

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:00, daytonalive83481
Ican determine the molar mass of an element by looking on the under the atomic mass for the element. for example the molar mass of phosphorus is 30.974 grams/mole. avogadro’s number tells me the amount of representative particles in 1 mole of any substance. this means 12.011 gram sample of carbon and a 32.0 gram sample of sulfur have the same number of atoms.
Answers: 1

Chemistry, 23.06.2019 00:00, familyk0jj3
How do you determine the percent yield of a chemical reaction
Answers: 1


Chemistry, 23.06.2019 01:30, elijahbebeastin
What are several ways to reduce the effect of friction
Answers: 2
You know the right answer?
calculate pressure exerted by 1.255 mol of CI2 in a volume of 5.005 L at a temperature 273.5 k using...
Questions in other subjects:

History, 03.03.2020 20:49

Mathematics, 03.03.2020 20:49




Social Studies, 03.03.2020 20:50



History, 03.03.2020 20:50

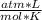 T= 273.5 K
T= 273.5 K