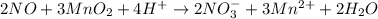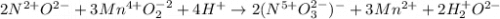Chemistry, 12.07.2021 18:40 am2garcia5
2NO + 3MnO2 + 4H â 2NO3- + 3Mn2 + 2H2O For the above redox reaction, assign oxidation numbers and use them to identify the element oxidized, the element reduced, the oxidizing agent and the reducing agent.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:00, advancedgamin8458
Consider the nuclear equation below. 239 > x + 4 he 94 2 what is x? 1.235 cm 96 2.243 u 92 3.235 u 92 4.243 cm 96
Answers: 2

Chemistry, 22.06.2019 02:20, kristieroth1
Compared with the freezing-point depression of a 0.01 m c6h12o6 solution, the freezing-point depression of a 0.01 m nacl solution is
Answers: 1

Chemistry, 22.06.2019 21:30, crystalbyrd79p8imrx
What is the effect of returning nuclear reactor cooling water back into bodies of water?
Answers: 3

You know the right answer?
2NO + 3MnO2 + 4H â 2NO3- + 3Mn2 + 2H2O For the above redox reaction, assign oxidation numbers and us...
Questions in other subjects:



Computers and Technology, 30.07.2019 12:00

Biology, 30.07.2019 12:00


History, 30.07.2019 12:00


History, 30.07.2019 12:00

Mathematics, 30.07.2019 12:00