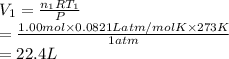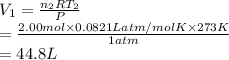Chemistry, 12.07.2021 05:40 mirzakasumovic8926
Consider an experimental run at 273 K where the initial number of moles (n1) is actually 1.00 mol, and the final number of moles (n2) is 2.00 mol. Use the simulation to find the volume (V1) of 1.00 mol of helium at 273 K, and calculate the final volume (V2). Express the volume to three significant figures, and include the appropriate units.

Answers: 2


Other questions on the subject: Chemistry



Chemistry, 23.06.2019 00:30, ejuarez2020
When a beta particle is emitted, the mass number of the nucleus a. decreases by one b. increases by one c. remains the same d. decreases by two
Answers: 2

Chemistry, 23.06.2019 11:30, melissalopez12
Place the following substances in order of ph from lowest ph to highest. a. neutral compounds, bases, acids b. acids, neutral compounds, bases c. bases, acids, neutral compounds d. bases, neutral compounds, acids
Answers: 1
You know the right answer?
Consider an experimental run at 273 K where the initial number of moles (n1) is actually 1.00 mol, a...
Questions in other subjects:

Social Studies, 06.03.2021 01:30

Mathematics, 06.03.2021 01:30

Mathematics, 06.03.2021 01:30



History, 06.03.2021 01:30


Physics, 06.03.2021 01:30

English, 06.03.2021 01:30

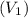 of 1.00 mol of helium at 273 K is 22.4 L and the final volume
of 1.00 mol of helium at 273 K is 22.4 L and the final volume 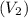 is 44.8 L.
is 44.8 L.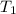 = 273 K,
= 273 K, 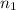 = 1.00 mol
= 1.00 mol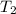 = 273 K,
= 273 K, 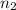 = 2.00 mol
= 2.00 mol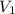 and
and 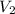 is calculated as follows.
is calculated as follows.