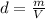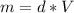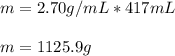Chemistry, 11.07.2021 20:40 Austin4094
Aluminum has a density of 2.70 g/mL. Calculate the mass (in grams) of a piece of aluminum having a volume of 417 mL .

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 10:20, blondielocks2002
Gwhich r group would most likely be found in a hydrophobic area of the tertiary structure of a globular protein? which r group would most likely be found in a hydrophobic area of the tertiary structure of a globular protein? −ch2−oh −ch2−o||c−nh2 −ch2−coo− −ch2−ch2−ch2−ch2−n+h3
Answers: 3
You know the right answer?
Aluminum has a density of 2.70 g/mL. Calculate the mass (in grams) of a piece of aluminum having a v...
Questions in other subjects:







English, 15.10.2019 02:30

Computers and Technology, 15.10.2019 02:30

History, 15.10.2019 02:30