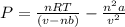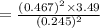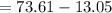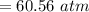Chemistry, 11.07.2021 19:10 joceeeeelyn2899
A 0.245-L flask contains 0.467 mol co2 at 159 °c. Caculate the pressure using Van der Walls equation

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:30, mazielynn84
How many molecules of sucrose c12h22o11 are there in 454 grams of sucrose
Answers: 1

Chemistry, 22.06.2019 07:30, gwenparks
Calculate the ratio of h+ ions to oh– ions at a ph = 7. find the concentration of h+ ions to oh– ions listed in table b of your student guide. then divide the h+ concentration by the oh– concentration. record this calculated ratio in table a of your student guide. compare your approximated and calculated ratios of h+ ions to oh– ions at a ph = 7. are they the same? why or why not? record your comparison in table a. what is the concentration of h+ ions at a ph = 7? mol/l what is the concentration of oh– ions at a ph = 7? mol/l what is the ratio of h+ ions to oh– ions at a ph = 7? : 1
Answers: 1

Chemistry, 22.06.2019 11:50, robert7248
The chemical bond connecting one nucleotide with the next one along the nucleic acid chain is called a
Answers: 3

Chemistry, 22.06.2019 12:00, carvajalj2520
Explain what happens at the saturation point when adding salt to water at room temperature.
Answers: 1
You know the right answer?
A 0.245-L flask contains 0.467 mol co2 at 159 °c. Caculate the pressure using Van der Walls equation...
Questions in other subjects:






World Languages, 07.04.2021 01:00


Mathematics, 07.04.2021 01:00


Mathematics, 07.04.2021 01:00