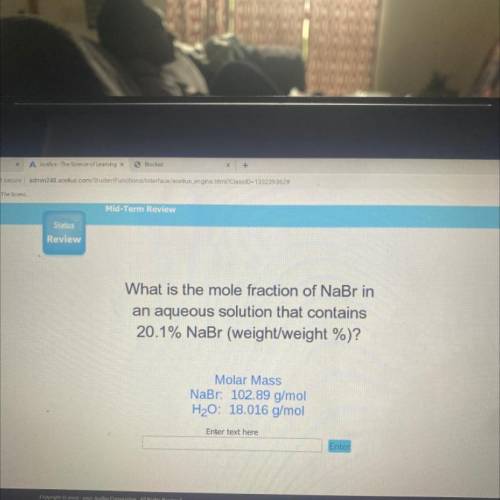
What is the mole fraction of NaBr in
an aqueous solution that contains
20.1% NaBr (weight/wei...


Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 14:30, Kiaraboyd9366
Select all of the statements which are true. electrons are located in shells or orbits around the atom. electrons orbit slowly around the atom. electrons travel in one flat path around the nucleus of an atom. the valence of an atom is determined by the number of electrons in the atom's outermost shell.
Answers: 1

Chemistry, 22.06.2019 23:30, emmalado45
If it is an isoelectronic series select true, if not select false. o2-, s2-, se2-, te2- na+, k+, rb+, cs+ n3-, p3-, as3-, sb3- ag, cd+, sn3+, sb4+ f-, cl-, br-, i- f-, ne, na+, mg2+ s2-, s, s6+
Answers: 1


Chemistry, 23.06.2019 23:10, makrosebud7821
What determines the types of chemical reactions that an atom participates in?
Answers: 1
You know the right answer?
Questions in other subjects:




Social Studies, 21.07.2019 02:30

Mathematics, 21.07.2019 02:30


Chemistry, 21.07.2019 02:30