

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 13:30, bryce99
In a ni-cd battery, a fully charged cell is composed of nickelic hydroxide. nickel is an element that has multiple oxidation states. assume the following proportions of the states: nickel charge proportions found 0 0.17 +2 0.3 +3 0.33 +4 0.5 (a) determine the mean of the nickel charge. enter the answer to 2 decimal places.(b) determine the cumulative distribution function of nickel charge.
Answers: 2

Chemistry, 22.06.2019 15:20, munziruddin204
Which description best characterizes the motion of particles in a solid?
Answers: 2

Chemistry, 22.06.2019 18:50, christhegreat1
Asample of tin (ii) chloride has a mass of 0.49 g. after heating, it has a mass of 0.41 g. what is the percent by mass of water in the hydrate? %
Answers: 1

Chemistry, 22.06.2019 19:00, montgomerykarloxc24x
Convert the temperature of dry ice, –77 ∞c, into degrees fahrenheit and kelvin.
Answers: 2
You know the right answer?
Using any data you can find in the ALEKS Data resource, calculate the equilibrium constant k at 25.0...
Questions in other subjects:

History, 04.08.2019 09:50

Mathematics, 04.08.2019 09:50

Chemistry, 04.08.2019 09:50

Chemistry, 04.08.2019 09:50






Mathematics, 04.08.2019 09:50

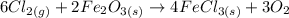
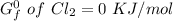
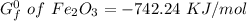
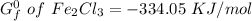
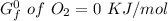
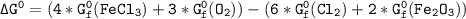
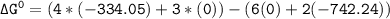
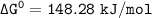
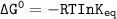
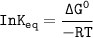
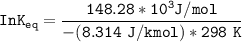
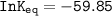

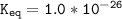 to 2 significant figures.
to 2 significant figures.

